Images are for illustration purposes only. Packaging may change from time to time and images on our website may or may not be updated.
Selection of 2 products from
£14.01 to £21.00
Target species:
For use in calves, pigs, chickens and turkeys. Indications for use, specifying the target species:For preventative medication the following programmes are recommended: 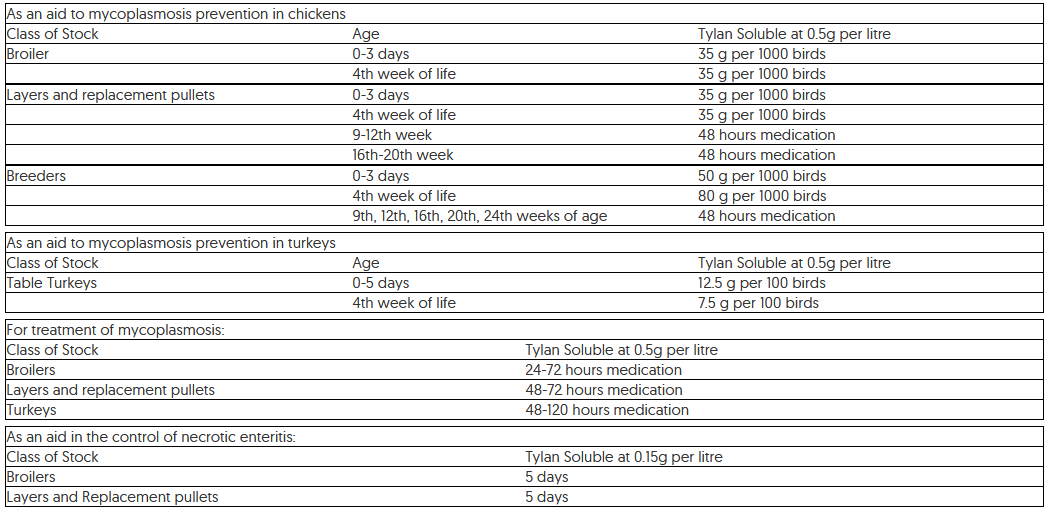
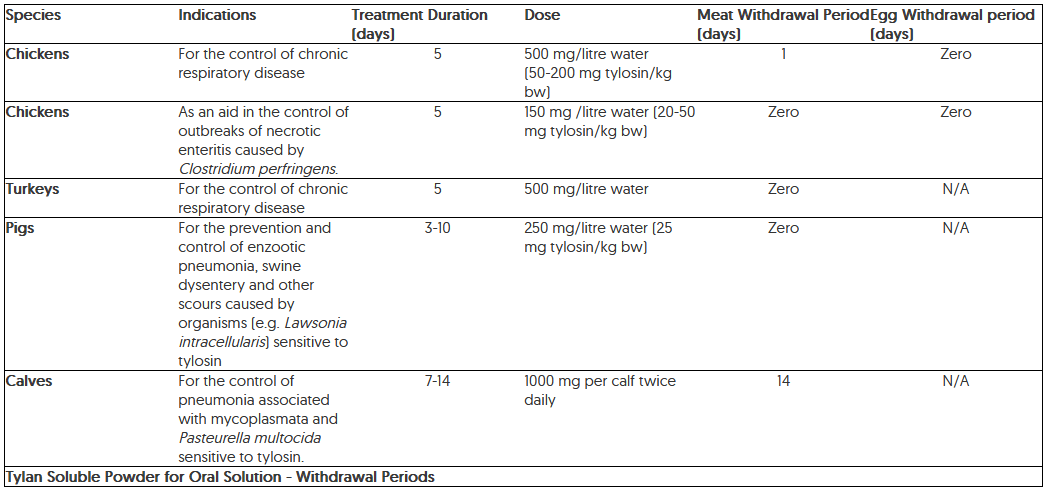
A medicated solution of drinking water should generally be administered until 24 hours after scouring or respiratory symptoms have ceased, normally 3-10 days. The diagnosis should be reviewed if there is no response after 5 days of medication. 
One gram of tylosin activity should be incorporated in milk or milk replacer twice daily for each calf. This should be continued for 7-14 days dependent on response. The intake of medicated water depends on the clinical condition of the animals. In order to obtain the correct dosage, the concentration of Tylan Soluble has to be adjusted accordingly.
There is no evidence of tylosin toxicity in animals, at dose rates of up to 1000 mg/kg.
All prices include VAT where applicable.
| Overall | |
| Value For Money | |
| Effectiveness | |
| Ease Of Use | |
| Absence Of Side Effects |
Only verified purchasers of this product can leave a review.
It worked
Customer recommends this product
My dog has been on Tylan periodically over the years for his tummy problem. I used viovet because the the new bottle prescribed by my vets had a use by date of October 2023. I got a prescription, use by date of 2025 and £30 cheaper
Customer does not recommend this product
This was suggested by our vet
Customer recommends this product
Sorted out my chickens respiratory problems
Customer recommends this product
I know that if i have a poorly hen with respiratory issues this is just the medicine to get her better without infecting her eggs
Customer recommends this product
Below are some recent questions we've received regarding Tylan Antibiotic, including answers from our team.
Samantha
Hi, if you give tylan to your chickens, can you eat the eggs.
The Tylan Soluble Powder has a zero day withdrawal period for eggs. This means you can eat the eggs during treatment.
Von
I have 2 pet chickens. My vet and I have discussed the various options I have but has not yet written me a script as she is unsure how long a tub this size would keep.
They do not routinely keep it at the practice and although she has said she will research the shelf life I figure as you sell it I will ask. Thank you
The shelf life for this product as per the datasheet is as follows:
Shelf life
Shelf-life of the veterinary medicinal product as packaged for sale: 3 years.
Shelf-life after dilution according to directions: 24 hours
We dispense what our wholesalers send us so cannot guarantee 3 year long expiries. It is likely that you will receive a shelf life shorter than 3 years when you purchase the medication.
Amy Danzl
What are the other ingredients in each of the solution and premix? Are there any additional active ingredients in either? Thanks!
The ingredients for these products are listed below, both only have tylosin as the active ingredient:
Tylan Premix:
Tylosin activity (as tylosin phosphate) 50 g per kg
Excipents; Soybean mill run, Isopar M
Tylan Soluble:
Tylosin 100g activity per bottle as Tylosin Tartrate.
No excipients
Karen moxon
Hi do you sell an oral version off tylan to be given in a syringe to chickens, the powder version is difficult to convert for oral syringing, I don’t like injecting in to my tiny chickens.
Regards Kare Moxon.
The Tylan Soluble and G50 Premix Powder are both for oral administration, as per the data sheet. This is a prescription medication so I would advise contacting your vet for advice on how to best administer it to your chickens.
andrea
Can this be used for sibo/sid for dogs ? My dog has been on metronizadole and hasn't had big improvement. I've read that tylan is now the drug of choice for this and ibd.
Tylan is one of several antibiotics used in this situation, but I would certainly not say that it is widely regarded as the antibiotic of choice. I am sure that some vets will think this and it might be very good in their experience, but not all vets would have the same opinion. In fact I have been to lectures on this recently and found that international speakers from the USA and UK tend to disagree, so there is not a general consensus on this point. I think that the choice of antibiotic is best left to your own vet. You can always discuss the matter fully with them, but if you are not happy with the choice of treatment your vet advises, I would wonder if you should see another vet. I really feel that the choice of prescription drugs should be led by your own vet who is familiar with your particular dog.
how much will I need
I have 10 chickens so how much Tulane will I need to treat them all? I have defiantly 1 chicken with an upper resperoitry infection (confirmed by the vet) not in the lungs just upper section
Since this is a prescription-only product, you would need to send us a prescription from your vet in order to buy it. Your vet should decide on how the treatment is given and put instructions on the prescription. As a guide, I would say that this is not generally used for just a few birds like you have. One small sachet could treat about 3,000 birds via the drinking water, so you would have to divide the contents of a sachet up very carefully to treat just 10 birds. Then you have to deal with the wasted product you do not need. It might be better for your vet to find a different treatment.