Images are for illustration purposes only. Packaging may change from time to time and images on our website may or may not be updated.
Selection of 3 products from
£2.46 to £117.96
Species: Dogs Therapeutic indication: Pharmaceuticals: Endoparasiticides: Anthelmintics for dogs Active ingredient: Milbemycin Oxime, Praziquantel Product:Milprazone Flavoured Tablets for Dogs Product index: Milprazon Tablets for Dogs Incorporating:Milprazon Flavoured Tablets for Small Dogs & Puppies Milprazon Flavoured Tablets for Dogs
Milprazon 2.5 mg/25 mg Tablets for Small Dogs and Puppies Weighing At Least 0.5 kg. Yellowish-white with brown spots, oval, biconvex tablets scored on one side. The tablets can be divided into equal halves. Milprazon 12.5 mg/125 mg Tablets for Dogs Weighing At Least 5 kg. Yellowish-white with brown spots, round, slightly biconvex tablets. 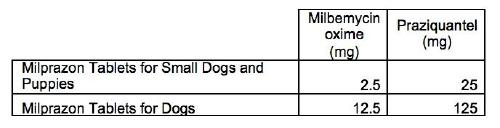
Treatment of mixed infections by adult cestodes and nematodes of the following species: - Cestodes: Dipylidium caninum, Taenia spp., Echinococcus spp., Mesocestoides spp. - Nematodes: Ancylostoma caninum, Toxocara canis,Toxascaris leonina,Trichuris vulpis Crenosoma vulpis (Reduction of the level of infection) Angiostrongylus vasorum (Reduction of the level of infection by immature adult (L5) and adult parasite stages), Thelazia callipaeda. The product can also be used in the prevention of heartworm disease (Dirofilaria immitis) if concomitant treatment against cestodes is indicated.
FOR ORAL ADMINISTRATION Dosage: Minimum recommended dose rate: 0.5 mg of milbemycin oxime and 5 mg of praziquantel per kg are given once orally. The product should be administered with or after some food. Depending on the bodyweight of the dog, the practical dosing is as follows: 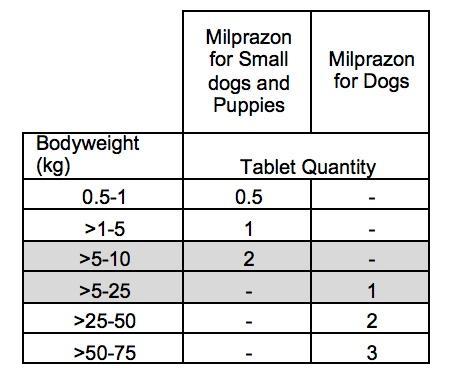 In cases when heartworm disease prevention is used and at the same time treatment against tapeworm is required, the product can replace the monovalent product for the prevention of heartworm disease. For treatment of Angiostrongylus vasorum infections, milbemycin oxime should be given four times at weekly intervals. It is recommended, where concomitant treatment against cestodes is indicated, to treat once with the product and continue with the monovalent product containing milbemycin oxime alone, for the remaining three weekly treatments. In endemic areas administration of the product every four weeks will prevent angiostrongylosis by reducing immature adult (L5) and adult parasite burden, where concomitant treatment against cestodes is indicated. For the treatment of Thelazia callipaeda, milbemycin oxime should be given in 2 treatments, seven days apart. Where concomitant treatment against cestodes is indicated, the product can replace the monovalent product containing milbemycin oxime alone. Use during pregnancy and lactation The product may be used in breeding dogs including pregnant and lactating bitches.
In cases when heartworm disease prevention is used and at the same time treatment against tapeworm is required, the product can replace the monovalent product for the prevention of heartworm disease. For treatment of Angiostrongylus vasorum infections, milbemycin oxime should be given four times at weekly intervals. It is recommended, where concomitant treatment against cestodes is indicated, to treat once with the product and continue with the monovalent product containing milbemycin oxime alone, for the remaining three weekly treatments. In endemic areas administration of the product every four weeks will prevent angiostrongylosis by reducing immature adult (L5) and adult parasite burden, where concomitant treatment against cestodes is indicated. For the treatment of Thelazia callipaeda, milbemycin oxime should be given in 2 treatments, seven days apart. Where concomitant treatment against cestodes is indicated, the product can replace the monovalent product containing milbemycin oxime alone. Use during pregnancy and lactation The product may be used in breeding dogs including pregnant and lactating bitches.
Milprazon 2.5 mg/25 mg Tablets for Small Dogs and Puppies Weighing At Least 0.5 kg. Do not use in puppies of less than 2 weeks of age and/or weighing less than 0.5 kg. Milprazon 12.5 mg/125 mg Tablets for Dogs Weighing At Least 5 kg. Do not use in dogs weighing less than 5 kg. Do not use in cases of hypersensitivity to the active substances or to any of the excipients. On very rare occasions, systemic signs (such as lethargy), neurological signs (such as muscle tremors and ataxia) and/or gastrointestinal signs (such as emesis, diarrhoea, anorexia and drooling) have been observed in dogs after administration of the combination of milbemycin oxime and praziquantel. Special precautions for use in animals Studies with milbemycin oxime indicate that the margin of safety in certain dogs of Collie or related breeds is less than in other breeds. In these dogs, the recommended dose should be strictly observed. The tolerance of the product in young puppies from these breeds has not been investigated. Clinical signs in Collies are similar to those seen in the general dog population when overdosed. Treatment of dogs with a high number of circulating microfilariae can sometimes lead to the appearance of hypersensitivity reactions, such as pale mucous membranes, vomiting, trembling, laboured breathing or excessive salivation. These reactions are associated with the release of proteins from dead or dying microfilariae and are not a direct toxic effect of the product. The use in dogs suffering from microfilaremia is thus not recommended. In heartworm risk-areas, or in the case it is known that a dog has been travelling to and from heartworm risk regions, before using the product, a veterinary consultation is advised to exclude the presence of any concurrent infestation of Dirofilaria immitis. In the case of a positive diagnosis, adulticidal therapy is indicated before administering the product. Echinococcosis represents a hazard for humans. In case of Echinococcosis, specific guidelines on the treatment and follow up and on the safeguard of persons have to be followed. Experts or institutes of parasitology should be consulted. No studies have been performed with severely debilitated dogs or individuals with seriously compromised kidney or liver function. The product is not recommended for such animals or only according to a benefit/risk assessment by the responsible veterinarian. In dogs less than 4 weeks old, tape worm infection is unusual. Treatment of animals less than 4 weeks old with a combination product may therefore not be necessary. Special precautions to be taken by the person administering the veterinary medicinal product to animals Wash hands after use. In the event of accidental ingestion of the tablets, particularly by a child, seek medical advice immediately and show the package leaflet or the label to the doctor. Interactions No interactions were observed when the recommended dose of the macrocyclic lactone selamectin was administered during treatment with the combination of milbemycin oxime and praziquantel at the recommended dose. In the absence of further studies, caution should be taken in the case of concurrent use of the product and other macrocyclic lactones. Also, no such studies have been performed with reproducing animals. Environmental Safety
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product should be disposed of in accordance with local requirements.
Store in the original package in order to protect from moisture. This veterinary medicinal product does not require any special temperature storage conditions. Halved tablets should be stored below 25°C in the original blister and be used for the next administration. Keep the blister in the outer carton Shelf life of the veterinary medicinal product as packaged for sale: 2 years Milprazon for Small Dogs and Puppies: Shelf life for halved tablets after first opening the immediate packaging: 6 months.
Legal category: POM-V
Aluminium blisters of 2 or 4. Packaged into boxes of 2, 4 or 48.
KRKA, d.d., Novo mesto Šmarješka cesta 6 8501 Novo mesto Slovenia
Pharmacotherapeutic group: Endectocides, Macrocyclic lactones, milbemycin, combinations ATCvet code: QP54AB51 Milbemycin oxime belongs to the group of macrocyclic lactones, isolated from the fermentation of Streptomyces hygroscopicus var. aureolacrimosus. It is active against mites, against larval and adult stages of nematodes as well as against larvae of Dirofilaria immitis. The activity of milbemycin is related to its action on invertebrate neurotransmission: Milbemycin oxime, like avermectins and other milbemycins, increases nematode and insect membrane permeability to chloride ions via glutamate-gated chloride ion channels (related to vertebrate GABAA and glycine receptors). This leads to hyperpolarisation of the neuromuscular membrane and flaccid paralysis and death of the parasite.
Milprazon Tablets for Small Dogs and Puppies Vm 01656/4075 Milprazon Tablets for Dogs Vm 01656/4076
GTIN description:Milprazon Tablets for Small Dogs and Puppies (2 tablet pack) GTIN:03838989649050 GTIN description:Milprazon Tablets for Small Dogs and Puppies (4 tablet pack) GTIN:03838989649074 GTIN description:Milprazon Tablets for Small Dogs and Puppies (48 tablet pack) GTIN:03838989649098 GTIN description:Milprazon Tablets for Dogs (2 tablet pack) GTIN:03838989649067 GTIN description:Milprazon Tablets for Dogs (4 tablet pack) GTIN:03838989649081 GTIN description:Milprazon Tablets for Dogs (48 tablet pack) GTIN:03838989649104
Species: Cats Therapeutic indication: Pharmaceuticals: Endoparasiticides: Anthelmintics for cats Active ingredient: Milbemycin Oxime, Praziquantel Product:Milprazone Tablets for Cats Product index: Milprazone Tablets for Cats Incorporating:Milprazone Tablets for Small Cats & Kittens Milprazone Tablets for Cats
Milprazon 4 mg/10 mg film-coated tablets for small cats and kittens weighing at least 0.5 kg. Brown yellow, oval, biconvex film-coated tablets with score line on one side. The tablets can be divided into equal halves. Milprazon 16 mg/40 mg film-coated tablets for cats weighing at least 2 kg. Brown red, oval, biconvex film-coated tablets with score line on one side. The tablets can be divided into equal halves. 
Treatment of mixed infections by immature and adult cestodes and nematodes of the following species: - Cestodes: Dipylidium caninum, Taenia spp., Echinococcus multilocularis - Nematodes: Ancylostoma tubaeforme,Toxocara cati Prevention of heartworm disease (Dirofilaria immitis) if concomitant treatment against cestodes is indicated.
FOR ORAL ADMINISTRATION Animals should be weighed to ensure accurate dosing. Minimum recommended dose rate: 2 mg of milbemycin oxime and 5 mg of praziquantel per kg are given orally as a single dose. The product should be administered with or after some food. Doing so ensures optimum protection against heartworm disease. Depending on the bodyweight of the cat, the practical dosing is as follows: 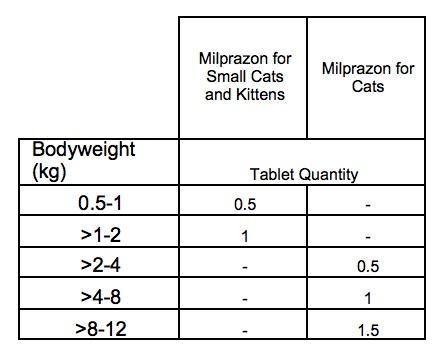 The product can be inserted into a programme for prevention of heartworm disease if at the same time treatment against tapeworms is indicated. The product has a duration of heartworm prevention of one month. For regular prevention of heartworm disease the use of a monosubstance is preferred. Use during pregnancy and lactation The veterinary medicinal product can be used in breeding cats including pregnant and lactating queens.
The product can be inserted into a programme for prevention of heartworm disease if at the same time treatment against tapeworms is indicated. The product has a duration of heartworm prevention of one month. For regular prevention of heartworm disease the use of a monosubstance is preferred. Use during pregnancy and lactation The veterinary medicinal product can be used in breeding cats including pregnant and lactating queens.
Milprazon 4 mg/10 mg film-coated tablets for small cats and kittens weighing at least 0.5 kg. Do not use in cats of less than 6 weeks of age and/or weighing less than 0.5 kg. Milprazon 16 mg/40 mg film-coated tablets for cats weighing at least 2 kg Do not use in cats weighing less than 2 kg. Do not use in case of hypersensitivity to the active substances or to any of the excipients. On very rare occasions, especially in young cats, systemic signs (such as lethargy), neurological signs (such as ataxia and muscle tremors) and/or gastrointestinal signs (such as emesis and diarrhoea) have been observed after administration of the combination milbemycin/praziquantel. Special precautions for use in animals It is recommended to treat all the animals living in the same household concomitantly. In order to develop an effective worm control programme local epidemiological information and the risk of exposure of the cat should be taken into account. When D. caninum infection is present, concomitant treatment against intermediate hosts, such as fleas and lice, should be considered to prevent re-infection. Parasite resistance to any particular class of anthelmintic may develop following frequent, repeated use of an anthelmintic of that class. No studies have been performed with severely debilitated cats or individuals with seriously compromised kidney or liver function. The product is not recommended for such animals or only according to a benefit/risk assessment by the responsible veterinarian. Special precautions to be taken by the person administering the veterinary medicinal product to animals In the event of accidental ingestion of the tablets, particularly by a child, seek medical advice immediately and show the package leaflet or the label to the doctor. Wash hands after use. Echinococcosis represents a hazard for humans. As Echinococcosis is a notifiable disease to the World Organisation for Animal Health (OIE), specific guidelines on the treatment and follow-up, and on the safeguard of persons, need to be obtained from the relevant competent authority. Overdose: In case of overdose, in addition to signs observed at the recommended dose, drooling may be observed. This sign will usually disappear spontaneously within a day. Interactions No interactions were observed when the recommended dose of the macrocyclic lactone selamectin was administered during treatment with the combination of milbemycin oxime and praziquantel at the recommended dose. In the absence of further studies, caution should be taken in the case of concurrent use of the product and other macrocyclic lactones. Also, no such studies have been performed with reproducing animals. Environmental Safety
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product should be disposed of in accordance with local requirements.The product should not enter water courses as this may be dangerous for fish and other aquatic organisms.
Store in the original package in order to protect from moisture. This veterinary medicinal product does not require any special temperature storage conditions. Halved tablets should be stored below 25°C in the original blister and be used for the next administration. Keep the blister in the outer carton Shelf life of the veterinary medicinal product as packaged for sale: 2 years Milprazon for Small Cats and Kittens: Shelf life for halved tablets after first opening the immediate packaging: 6 months. Milprazon for Cats: Shelf life for halved tablets after first opening the immediate packaging: 6 months.
Legal category: POM-V
Aluminium blisters of 2 or 4. Packaged into boxes of 2, 4 or 48. Not all pack sizes may be marketed.
KRKA, d.d., Novo mesto Šmarješka cesta 6 8501 Novo mesto Slovenia
Pharmacotherapeutic group: Endectocides, Macrocyclic lactones, milbemycin, combinations ATCvet code: QP54AB51 Milbemycin oxime belongs to the group of macrocyclic lactones, isolated from the fermentation of Streptomyces hygroscopicus var. aureolacrimosus. It is active against mites, against larval and adult stages of nematodes as well as against larvae of Dirofilaria immitis. The activity of milbemycin is related to its action on invertebrate neurotransmission: Milbemycin oxime, like avermectins and other milbemycins, increases nematode and insect membrane permeability to chloride ions via glutamate-gated chloride ion channels (related to vertebrate GABAA and glycine receptors). This leads to hyperpolarisation of the neuromuscular membrane and flaccid paralysis and death of the parasite. Praziquantel is an acylated pyrazino-isoquinoline derivative. Praziquantel is active against cestodes and trematodes. It modifies the permeability for calcium (influx of Ca2+) in the membranes of the parasite inducing an imbalance in the membrane structures, leading to membrane depolarisation and almost instantaneous contraction of the musculature (tetany), rapid vacuolization of the syncytial tegument and subsequent tegumental disintegration (blebbing), resulting in easier expulsion from the gastrointestinal tract or death of the parasite.
Milprazon Tablets for Small Cats and Kittens Vm 01656/4084 Milprazon Tablets for Cats Vm 01656/4085
GTIN description:Milprazon Tablets for Small Cats and Kittens(2 tablets) GTIN:03838989652043 GTIN description:Milprazon Tablets for Small Cats and Kittens(4 tablets) GTIN:03838989652050 GTIN description:Milprazon Tablets for Small Cats and Kittens(48 tablets) GTIN:03838989652067 GTIN description:Milprazon Tablets for Cats (2 tablets) GTIN:03838989650711 GTIN description:Milprazon Tablets for Cats (4 tablets) GTIN:03838989650728 GTIN description:Milprazon Tablets for Cats (48 tablets) GTIN:03838989650735
All prices include VAT where applicable.
| Overall | |
| Effectiveness | |
| Ease Of Use | |
| Value For Money | |
| Absence Of Side Effects |
Only verified purchasers of this product can leave a review.
Easy to administer and effective treatment for worm control in dogs
Customer recommends this product
Effective
Customer recommends this product
Below are some recent questions we've received regarding Milprazon Film-Coated Tablets for Cats & Dogs, including answers from our team.
Em
My rescue dog had his first tablet yesterday and I have since seen roundworm in his faeces. Should I give his next tablet sooner than 1 month to make sure the worm cycle gets stopped?
This is a prescription only wormer so any dose changes will need to be run past your vet. They will be able to advise on whether you need to give another dose sooner.
Diane Patterson
How much Milprazon do I give my 6.5 month puppy weighs 26.25 kg Thanks
Your 26.25kg dog will need 2 x Milprazon for Adult Dogs according to the datasheet. The film-coated adult tablets are now discontinued though and are no longer available, replaced by the Milprazon Chewable Tablets.
This is a prescription only medication so you should contact your vet regarding dosing as they will be prescribing it to you.
Vanessa
Milprazon states 2.5mg/25mg for small dogs/puppies. I have 5 month old lab weighs 16kg. What age are you classing puppy and when do I change him to 12.5mg/125mg tablets?
These tablets are dosed by weight not by age. If your dog is over 5kg but under 25kg then the dose according to the datasheet is one adult 12.5mg/125mg tablet. This is a prescription only medication so you should contact your prescribing vet to discuss doseage.
Linz
My dog is 2 years old and his weight is 5.3kg . He has been sick when given 2 tablets for his 3monthly worming can I cut one in half and give him one and a half rather than 2. Thanks
This may be possible but it would be an off license use of the medication. Unfortunately we cannot provide off license advice, it is recommended to discuss this further with your prescribing vet who can look into this for you.
Jess
Does this product specifically treat lung worm in dogs
Yes, Milprazon is licensed to treat Angiostrongylus vasorum (AKA lungworm) according to the datasheet.
Karen
Hi
My 8month old puppy refuses to swallow milprazon and even when we try to disguise it in his favourite foods; he just spits it out. We finally got him to swallow the tablet however it ended up in little pieces. Will this affect its effectiveness?
Thanks
Yes these tablets can be split and crushed if necessary. It should not change the efficacy of the medication.
Tracy Burden
How often can milprazon be given to my dog? My vet says every 6 months but I think she needs worming more frequently
Milprazon contains milbemycin oxime and praziquantel. This combination of ingredients can be safely given more often than every 6 months. It is recommended to give this monthly if you are wanting to prevent against adult lungworm infestation. Your vet may be advising every 6 months though as you have alternate lungworm coverage, I advise discussing this with them.